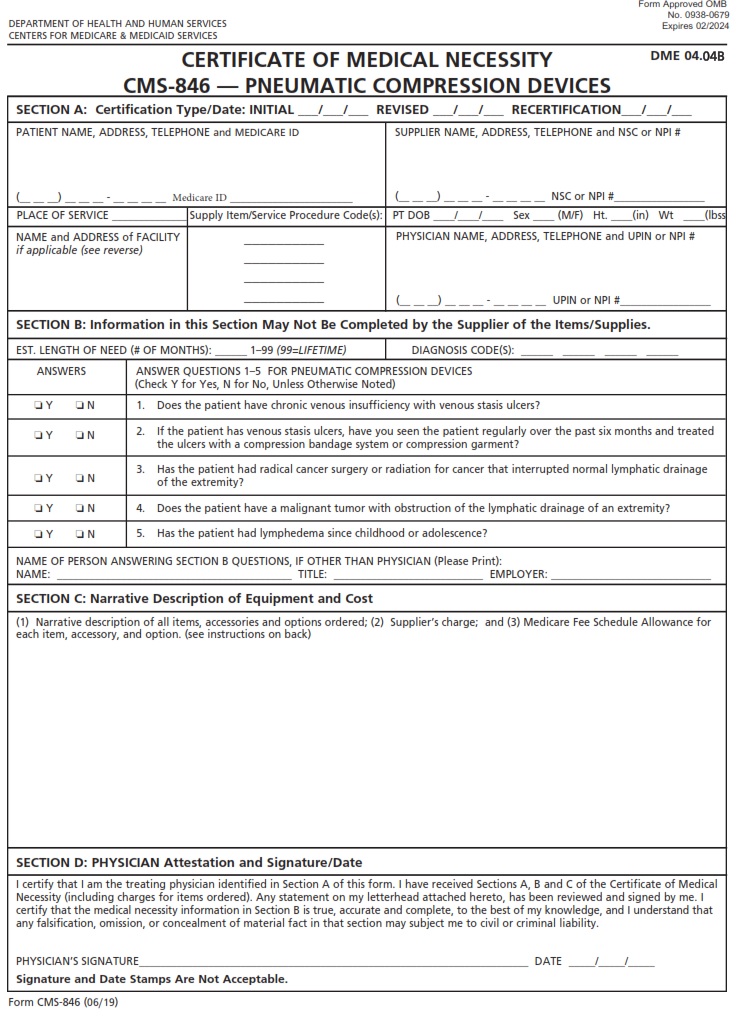
CMSFORM.ORG – CMS 846 – Certificate of Medical Necessity – Pneumatic Compression Devices DME 04.04B – As the healthcare landscape continues to evolve, the importance of precise documentation and justification for medical equipment becomes increasingly crucial. One such document that plays a key role in ensuring patients receive necessary devices is CMS 846 – Certificate of Medical Necessity for Pneumatic Compression Devices DME 04.04B. This seemingly mundane form holds the power to impact patient care significantly, serving as a bridge between healthcare providers and insurance companies in the quest for optimal treatment outcomes.
Imagine a world where medical necessity is not just a bureaucratic hurdle but a pivotal tool in advocating for patient well-being. With pneumatic compression devices becoming essential aids in managing various health conditions, understanding and mastering the intricacies of CMS 846 can unlock doors to improved access to vital equipment for those who need it most. In this article, we delve into the nuances of this often overlooked certificate, exploring its implications on patient care and shedding light on how it shapes the landscape of durable medical equipment provision.
Download CMS 846 – Certificate of Medical Necessity – Pneumatic Compression Devices DME 04.04B
| Form Number | CMS 846 |
| Form Title | Certificate of Medical Necessity – Pneumatic Compression Devices DME 04.04B |
| Published | 2017-02-01 |
| O.M.B. | 0938-0679 |
| File Size | 3 KB |
What is a CMS 846?
A CMS 846 form, also known as a Certificate of Medical Necessity, plays a crucial role in the approval process for Pneumatic Compression Devices under DME 04.04B guidelines. This document serves as a validation from healthcare providers that the prescribed equipment is essential for managing a patient’s medical condition effectively. By detailing the specific medical needs and conditions requiring pneumatic compression therapy, the CMS 846 form ensures that patients receive appropriate care tailored to their individual requirements.
Moreover, submitting a CMS 846 form not only facilitates insurance coverage but also demonstrates compliance with Medicare regulations governing Durable Medical Equipment. Healthcare professionals must accurately complete this form to justify the necessity of Pneumatic Compression Devices for their patients. As such, understanding and utilizing the CMS 846 documentation process is vital for ensuring uninterrupted access to critical medical equipment while adhering to regulatory standards set by Medicare and other insurers.
Where Can I Find a CMS 846?
CMS 846 forms, essential for obtaining pneumatic compression devices under DME 04.04B, can be sourced from various avenues. Healthcare providers and Durable Medical Equipment suppliers are primary sources for acquiring the CMS 846 form. These entities often possess up-to-date versions of the form that align with regulatory requirements and streamline the process of obtaining necessary medical equipment.
For individuals looking to acquire a CMS 846 form independently, exploring official government websites such as Medicare.gov can be fruitful. Additionally, some healthcare facilities provide online resources where patients can access and download CMS 846 forms directly. By leveraging these multiple pathways, individuals seeking pneumatic compression devices can efficiently navigate the documentation process and ensure timely compliance with medical necessity requirements.
CMS 846 – Certificate of Medical Necessity – Pneumatic Compression Devices DME 04.04B
When it comes to the CMS 846 form for pneumatic compression devices, precision and thoroughness are crucial. This certificate of medical necessity plays a significant role in ensuring that patients receive the durable medical equipment required for their healthcare needs. Healthcare providers completing this form must accurately detail the patient’s condition, treatment plan, and how a pneumatic compression device will benefit them in managing their condition. Moreover, highlighting the medical necessity of such devices can expedite insurance coverage approval processes and ensure timely access to vital equipment for patients.
The intricate nature of DME reimbursement highlights the importance of proper documentation through forms like CMS 846. Medical providers must navigate complex coding requirements and specific criteria to prove that pneumatic compression devices are not only beneficial but also essential in managing a patient’s health condition. Effective communication between healthcare professionals, patients, and insurance companies is key in streamlining the process and ensuring that patients receive prompt access to necessary medical equipment. By understanding the significance of CMS 846 in justifying medical necessity for pneumatic compression devices, stakeholders can work together effectively towards optimal patient care outcomes.