CMSFORM.ORG – CMS 1557 – SURVEY REPORT FORM – CLIA – In the intricate world of healthcare regulation, one acronym stands out amidst the sea of jargon: CMS 1557. This seemingly innocuous combination of letters and numbers holds immense significance in shaping the landscape of medical practices across the United States. But what exactly does it entail? Enter the Survey Report Form – CLIA, a crucial component within the realm of CMS 1557 that delves into the heart of compliance and quality assurance within clinical laboratories. As we navigate through this labyrinthine intersection of regulations and standards, let us unravel the mysteries behind CMS 1557 – Survey Report Form – CLIA, shedding light on its impact and implications for both providers and patients alike. Join us on a journey through this essential aspect of modern healthcare governance, where every box checked carries profound implications for the delivery of care.
Download CMS 1557 – SURVEY REPORT FORM – CLIA
| Form Number | CMS 1557 |
| Form Title | SURVEY REPORT FORM – CLIA |
| Published | 2021-02-01 |
| O.M.B. | 0938-0544 |
| File Size | 1 MB |
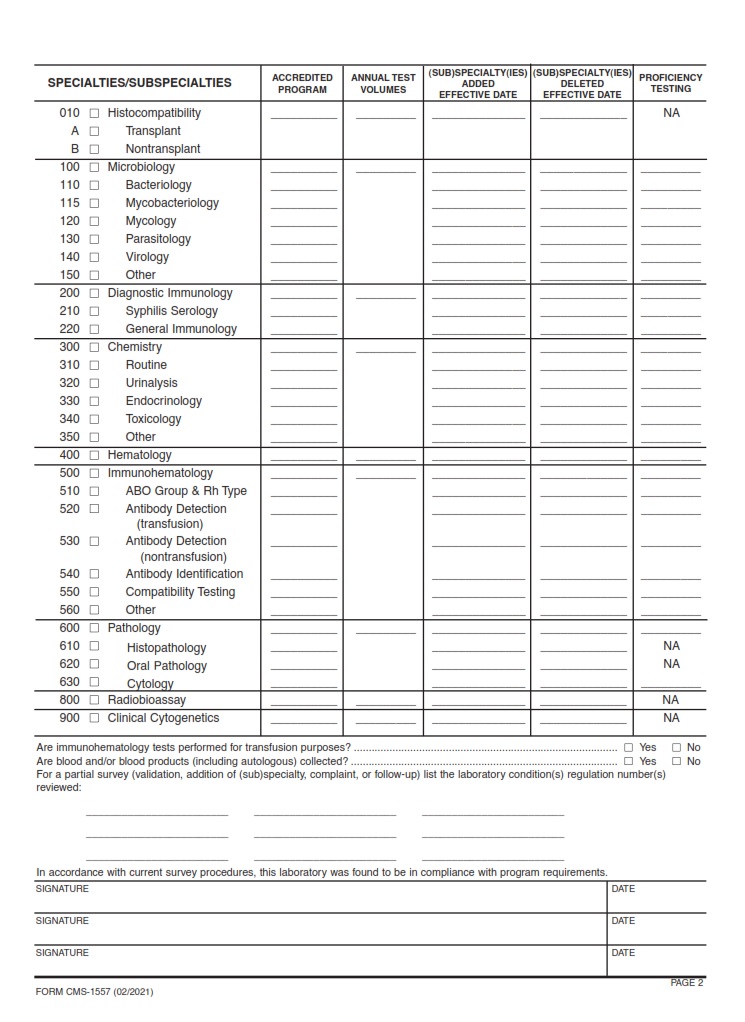
What is a CMS 1557?
CMS-1557 is an essential form used to collect data for laboratory testing facilities under the Clinical Laboratory Improvement Amendments (CLIA) program. This form allows these facilities to report important information regarding the tests they perform, quality control measures, and personnel qualifications. By ensuring compliance with CLIA regulations, CMS-1557 helps maintain high standards of quality and safety in clinical laboratories.
Moreover, CMS-1557 serves as a valuable tool for regulatory oversight by providing insight into the inner workings of laboratory testing facilities. Through this form, health authorities can monitor and assess the performance of these facilities to safeguard public health. The data collected through CMS-1557 plays a crucial role in identifying areas for improvement and ensuring that patients receive accurate and reliable test results from certified laboratories.
Where Can I Find a CMS 1557?
The CMS 1557 form, also known as the Survey Report Form for Clinical Laboratory Improvement Amendments (CLIA), is a crucial document in ensuring quality healthcare services. If you are searching for a CMS 1557 form, your first point of contact should be the official website of the Centers for Medicare & Medicaid Services (CMS). Here, you can navigate to the Forms section and search for CMS 1557 to access and download the form directly.
In addition to the CMS website, you can also inquire about obtaining a CMS 1557 form from your healthcare provider or local CLIA laboratory. These organizations may have physical copies available or be able to guide you on where to obtain one. It’s essential to have this form readily accessible as it plays a significant role in maintaining compliance with CLIA regulations and ensuring high standards in clinical laboratory practices.
CMS 1557 – SURVEY REPORT FORM – CLIA
CMS 1557, also known as the Survey Report Form – CLIA, plays a crucial role in ensuring compliance with federal regulations and maintaining high standards of patient care in healthcare facilities. This form serves as a comprehensive tool for surveyors to assess laboratories’ adherence to the Clinical Laboratory Improvement Amendments (CLIA) guidelines, which are designed to promote accuracy and quality in laboratory testing processes.
By meticulously evaluating various aspects of laboratory operations, such as personnel qualifications, quality control measures, and proficiency testing protocols, the CMS 1557 form enables surveyors to identify areas for improvement and provide recommendations for enhancing overall performance. It serves as a valuable resource for both healthcare providers and regulatory bodies alike, facilitating ongoing monitoring and evaluation of lab practices to ensure optimal patient outcomes.
Understanding the significance of the CMS 1557 – Survey Report Form – CLIA is essential for healthcare professionals seeking to maintain compliance with CLIA regulations and deliver superior laboratory services. By leveraging this tool effectively, organizations can streamline their accreditation processes, enhance quality assurance measures, and ultimately elevate the standard of care provided to patients.
CMS 1557 Example